Connecting packaging to patients™
99K
HAI Deaths in the US. A surprisingly little talked about topic in general media.
4M
28.4
Billion dollars cost directly to hospitals caused by HAIs in the United States each year.
3rd
Our Story and mission for
Improved Patient safety…
More than just a mission of awareness, we bring action…
Sterile packaging support
The SterileAware® Podcast goes live
The SterileAware® Podcast will provide insight into the Hospital born infection story with interviews with leading experts in HAI’s. The SterileAware® Podcast is hosted by our founder Charlie Webb, an award-winning podcaster and host of the popular Healthcare packaging Podcast “SPOT radio”. Stay up to date with the SterileAware® story.
Listen now
Beyond medical device pouch sealer
For 27 years we have provided the industries the most advanced medical device pouch sealing machinery. Beyond these machines, we also offer a host of medical pouch testing systems such as dye penetration kits and pouch testing machinery. We deliver our medical device pouch sealers with full calibration certification from our ISO/IEC 17025 accredited laboratory in order to fast-track your medical packaging validation.
Innovating for safer device delivery
Innovation does not always support a business model. Through the Van der Stähl Scientific product development cycle we have created needed innovations that has not improved our bottom line. The SterileAware® value means we will innovate with the goal of improved patient safety before we put it under the litmus test of profitability. The future of SterileAware® will include a panel of medical device packaging innovators.
Pouch testing and Calibration Labs
Most providers of medical device packaging machinery require you to send your machinery to a third-party lab for calibration. We support our medical device manufacturers with two ISO 17015 accredited laboratories that create a seamless connection between the machine and your regulatory needs. The SterileAware® value means purchasing critical packaging machinery from firms that support your regulatory requirement.
A total approach to healthcare packaging
SterileAware® takes a fresh approach to solving healthcare packaging problems with education, awareness, and innovation.
A total approach to healthcare packaging
SterileAware™ takes a fresh approach to solving healthcare packaging problems with education, awareness, and innovation.

The Heart of SterileAware®
SterileAware® is more than just awareness, we are about medical packaging solutions. Our scope includes everything from packaging machinery, validation services, testing, and inspection products to pouch testing and machine calibration services from our ISO-accredited laboratories.
When device contamination hits home
Charlie and Lisa Webb’s lives were shaken in the summer of 2022 when Charlie developed an infection from a contaminated scope that may have contributed to his near-deadly bleeding ulcer. The couple founded SterileAware® to help safeguard others from HAI (hospital-acquired infections) by creating awareness and solutions for healthcare packaging. We invite you to explore our page and learn about all the ways we will serve.
Healthcare packaging scholarships
Our goal in the coming years is to provide scholarships specific to healthcare packaging to foster enrollment in this discipline. If we are going to meet the growing demands of delivering medical devices safe and sterile to the patient, we will need to enlist more talent for this growing segment. We are also evaluating incentives to provide pivot enticement for packaging engineers to move into this growing space.
Our growing and changing medical device packaging marketplace.
As the healthcare packaging industry grows and shifts in potentially unexpected directions, how will we respond as an industry? Perhaps the path forward is heightened collaboration with medical device manufacturers, vendors, and users. “Speed to Care” will need to be the new value and mantra if we are to meet our call to action in Healthcare Packaging.
%
Annual Growth
Forecasted for the global sterile
medical packaging market.
%
Ethylene Sterilized
Estimated for medical devices
sterilized in the United States.
Meet Lisa our mission coordinator
The SterileAware® mission is all about medical device packaging systems, education, innovation, support, and awareness. Lisa Wassberg will head our “Awareness Campaign” as well as oversee the development of educational tools for sterile packaging. As the general manager of Van der Stähl Scientific, she will be calling on her expertise in making things happen quickly for device makers. Lisa has over 16 years of experience working with all the major US medical device manufacturers as well as hundreds of device startups and leads our packaging scholarship program. Expect amazing things to come to the Healthcare packaging space from the SterileAware® mission.
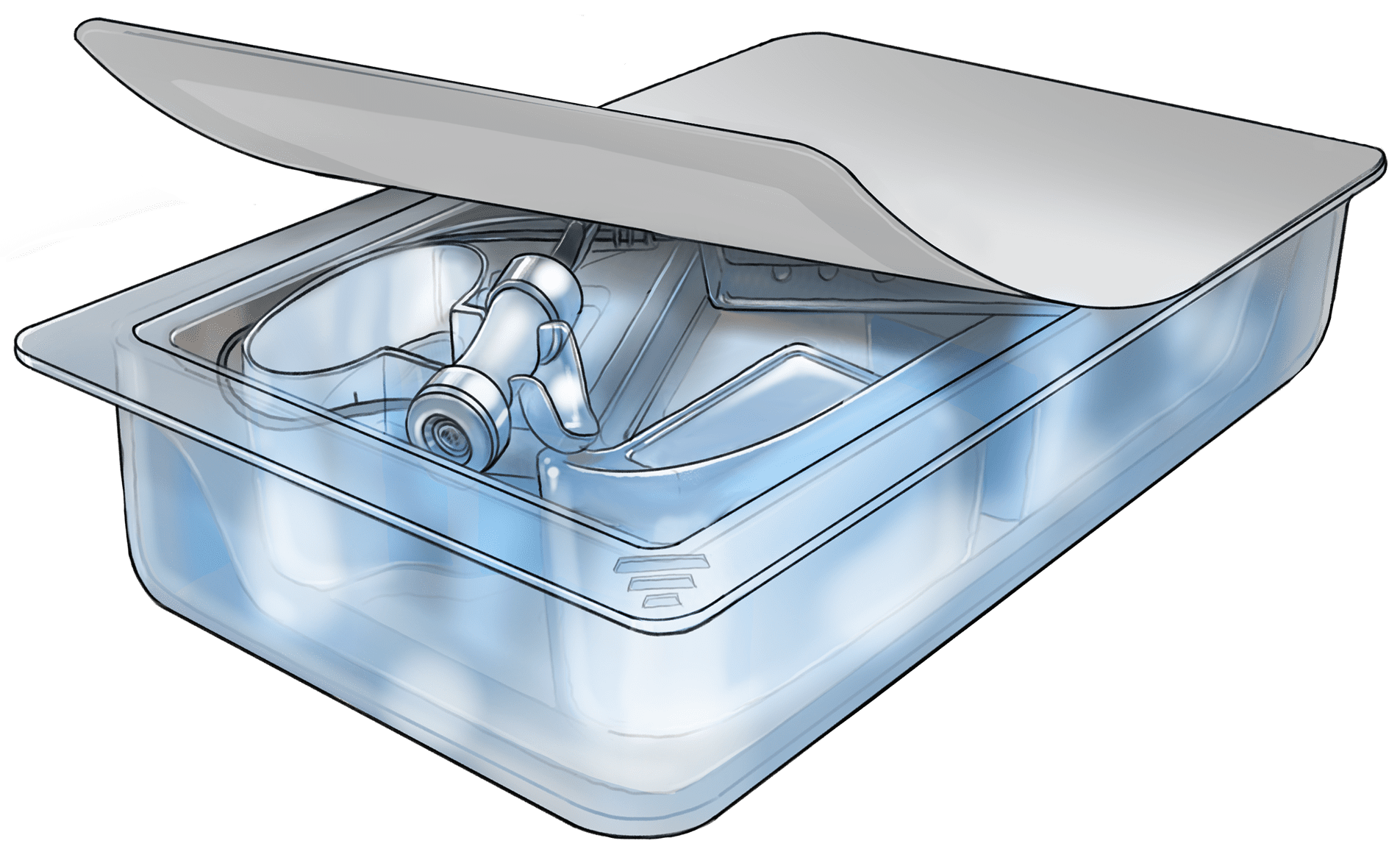
SterileAware® Certified Systems
Medical device packaging validation can be challenging as regulatory demands continue to swell. With our SterileAware® approved systems, we will provide medical device companies a head start with multiple ISO-11607-ready systems. Under the Superstars in Sterile Awareness™ model we will encourage device makers to use approved packaging systems to assure compliance to the ISO-17025 directive. Over the last 27 years, we have heard frustrations from medical device manufacturers that the pouch sealers they purchase did not meet the demanding rigors of the ISO 11607 standard. So why risk a 483 with non-conforming equipment? So just look for the “SterileAware® Approved System INSIDE” label and begin your medical device packaging journey with confidence.
Meet Lisa our mission coordinator
The SterileAware® mission is all about medical device packaging systems, education, innovation, support, and awareness. Lisa Wassberg will head our “Awareness Campaign” as well as oversee the development of educational tools for sterile packaging. As the general manager of Van der Stähl Scientific, she will be calling on her expertise in making things happen quickly for device makers. Lisa has over 16 years of experience working with all the major US medical device manufacturers as well as hundreds of device startups and leads our packaging scholarship program. Expect amazing things to come to the Healthcare packaging space from the SterileAware® mission.
SterileAware® Certified Systems
Medical device packaging validation can be challenging as regulatory demands continue to swell. With our SterileAware® approved systems, we will provide medical device companies a head start with multiple ISO-11607-ready systems. Over the last 27 years, we have heard frustrations from medical device manufacturers that the pouch sealers they purchase did not meet the demanding rigors of the ISO 11607 standard. So why risk a 483 with non-conforming equipment? Validation should not be overly challenging when you purchase the correct equipment for the task. Just look for the “SterileAware® Approved System INSIDE” label and begin your medical device packaging journey with confidence.

Celebrating with the Superstars in Sterile Awareness™ Award
|
SterileAware® means understanding every touch and stopping point on a patient's journey through a healthcare facility. From materials, machines, methods, and human connection we must remain mindful of how we connect to patient safety. As this emerging “zero failure” in sterile processing, packaging, and patient connection value continues to bloom, we will be here to support this growing movement, to keep us all Sterile Aware…"
-Charlie Webb CPPL (founder)
Empirical packaging data from our accredited Laboratory
SterileAware® will provide medical device packaging testing services to help our customers build a stronger medical device packaging validation. In cooperation with our parent company Van der Stahl Scientific, our customers can develop their design of experiments from solid data developed from our award-winning lab. Our ISO 17025 laboratory also provides comprehensive medical device packaging machine calibration to provide system traceability for your packaging validation.

SterileAware News
No-cost education
SterileAware® will champion sterile packaging awareness through a host of media events as well as provide free educational tools.
Innovations that matter
Dated inadequate packaging systems risk patient safety due to sterility loss. Explore our emerging healthcare packaging technology.