Machinery for your zero defect value…
SterileAware® takes a fresh approach to solving healthcare packaging problems with education, awareness, and innovation.

Machinery for your zero defect value…
A single seal failure on your medical pouch is unacceptable. Choose packaging machinery with a focus on patient safety.

Great things coming soon from the SterileAware® innovation lab…
Industry connecting to a cause
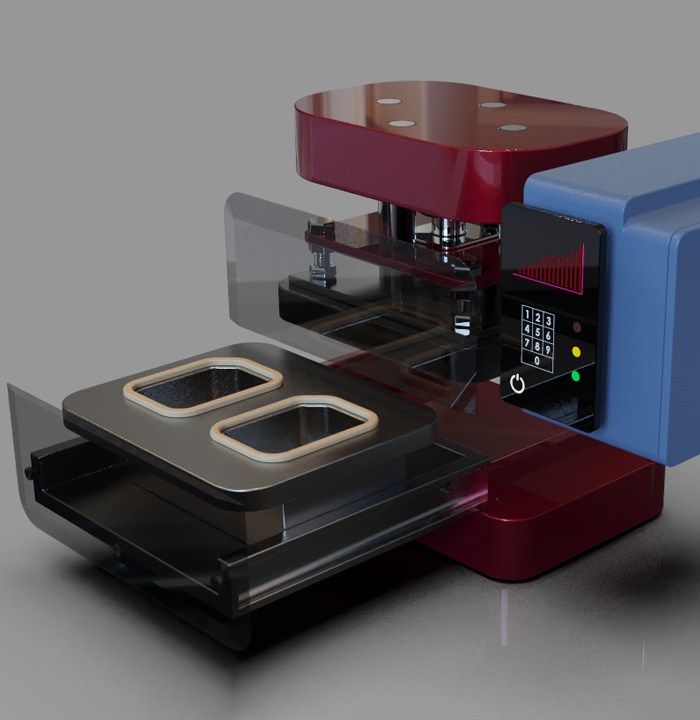
Automated Seal Testing
Human loading behavior can cause data scatter when performing the ASTM F-88 seal strength test. The PTT-500-AV is designed to accurately cut and load medical pouch seal samples to assure seal to seal test accuracy. The systems automation means no complicated training is needed and greatly encourages hourly testing to ensure that there is no processed drift.
Solutions born from collaboration
Our “innovation partners program” is an innovation incubator designed to imagine new technologies that will serve to mitigate the spread of hospital associate infections. Improved packaging and medical device pouch testing systems will be our first target as we can leverage our 27 years of experience. Stay tuned as we take aim at patient safety.
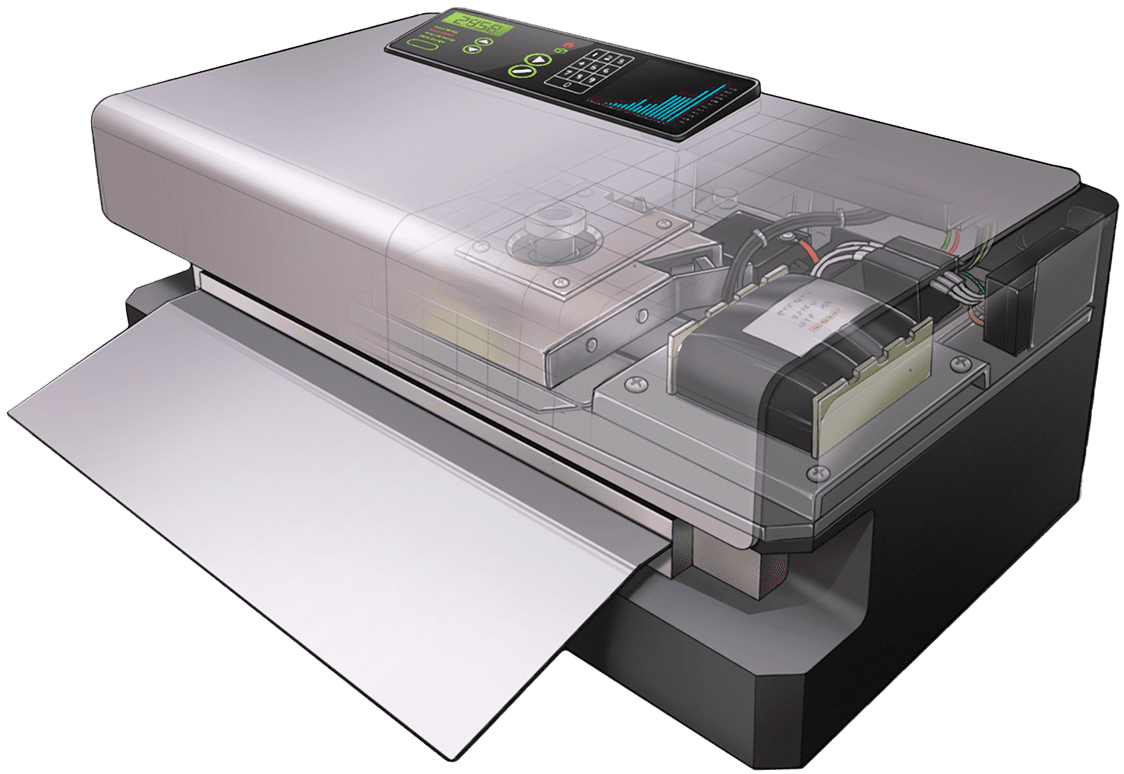
MTS-2500 Tray Sealing System
MTS-2500 Tray Sealing System
Same proven pouch sealing platform with an advanced microprocessor.
Same proven pouch sealing platform with an advanced microprocessor.
With our 26-year partnership with Fuji we have brought the MS-350 medical pouch sealer to thousands of cleanrooms worldwide. Through our user network, billions of sterile barrier seals have been created to deliver medical devices safe and sterile to the point of use. As we look forward to the next generation of this sturdy proven platform, we see exciting new ways to enhance user experience and system capability.
A total approach to healthcare packaging
SterileAware® takes a fresh approach to solving healthcare packaging problems with education, awareness, and innovation.
A total approach to healthcare packaging
SterileAware™ takes a fresh approach to solving healthcare packaging problems with education, awareness, and innovation.

The Heart of SterileAware®
SterileAware® is more than just awareness, we are about medical packaging solutions. Our scope includes everything from packaging machinery, validation services, testing, and inspection products to pouch testing and machine calibration services from our ISO-accredited laboratories.
When device contamination hits home
Charlie and Lisa Webb’s lives were shaken in the summer of 2022 when Charlie developed an infection from a contaminated scope that may have contributed to his near-deadly bleeding ulcer. The couple founded SterileAware® to help safeguard others from HAI (hospital-acquired infections) by creating awareness and solutions for healthcare packaging. We invite you to explore our page and learn about all the ways we will serve.
Healthcare packaging scholarships
Our goal in the coming years is to provide scholarships specific to healthcare packaging to foster enrollment in this discipline. If we are going to meet the growing demands of delivering medical devices safe and sterile to the patient, we will need to enlist more talent for this growing segment. We are also evaluating incentives to provide pivot enticement for packaging engineers to move into this growing space.
Empirical packaging data from our accredited Laboratory
SterileAware® will provide medical device packaging testing services to help our customers build a stronger medical device packaging validation. In cooperation with our parent company Van der Stahl Scientific, our customers can develop their design of experiments from solid data developed from our award-winning lab. Our ISO 17025 laboratory also provides comprehensive medical device packaging machine calibration to provide system traceability for your packaging validation.

Meet Lisa our mission coordinator
The SterileAware® mission is all about medical device packaging systems, education, innovation, support, and awareness. Lisa Wassberg will head our “Awareness Campaign” as well as oversee the development of educational tools for sterile packaging. As the general manager of Van der Stähl Scientific, she will be calling on her expertise in making things happen quickly for device makers. Lisa has over 16 years of experience working with all the major US medical device manufacturers as well as hundreds of device startups and leads our packaging scholarship program. Expect amazing things to come to the Healthcare packaging space from the SterileAware® mission.
SterileAware® Certified Systems
Medical device packaging validation can be challenging as regulatory demands continue to swell. With our SterileAware® approved systems, we will provide medical device companies a head start with multiple ISO-11607-ready systems. Under the Superstars in Sterile Awareness™ model we will encourage device makers to use approved packaging systems to assure compliance to the ISO-17025 directive. Over the last 27 years, we have heard frustrations from medical device manufacturers that the pouch sealers they purchase did not meet the demanding rigors of the ISO 11607 standard. So why risk a 483 with non-conforming equipment? So just look for the “SterileAware® Approved System INSIDE” label and begin your medical device packaging journey with confidence.
Meet Lisa our mission coordinator
The SterileAware® mission is all about medical device packaging systems, education, innovation, support, and awareness. Lisa Wassberg will head our “Awareness Campaign” as well as oversee the development of educational tools for sterile packaging. As the general manager of Van der Stähl Scientific, she will be calling on her expertise in making things happen quickly for device makers. Lisa has over 16 years of experience working with all the major US medical device manufacturers as well as hundreds of device startups and leads our packaging scholarship program. Expect amazing things to come to the Healthcare packaging space from the SterileAware® mission.
SterileAware® Certified Systems
Medical device packaging validation can be challenging as regulatory demands continue to swell. With our SterileAware® approved systems, we will provide medical device companies a head start with multiple ISO-11607-ready systems. Over the last 27 years, we have heard frustrations from medical device manufacturers that the pouch sealers they purchase did not meet the demanding rigors of the ISO 11607 standard. So why risk a 483 with non-conforming equipment? Validation should not be overly challenging when you purchase the correct equipment for the task. Just look for the “SterileAware® Approved System INSIDE” label and begin your medical device packaging journey with confidence.
Our growing and changing medical device packaging marketplace.
As the healthcare packaging industry grows and shifts in potentially unexpected directions, how will we respond as an industry? Perhaps the path forward is heightened collaboration with medical device manufacturers, vendors, and users. “Speed to Care” will need to be the new value and mantra if we are to meet our call to action in Healthcare Packaging.
%
Annual Growth
Forecasted for the global sterile
medical packaging market.
%
Ethylene Sterilized
Estimated for medical devices
sterilized in the United States.
Innovation past and present
The SterileAware™ supports the medical device packaging universe including everything from packaging machinery, validation services, testing, and inspection products as well as pouch testing and machine calibration services from our ISO 17025 accredited laboratories.
Sealing, Inspection and Testing
Our MS-451 medical device packaging system has been called the Swiss Army Knife of medical device packaging. Medical device pouch testing and inspection are so valuable to the process of sterile packaging we have incorporated a precision tensile/peel strength testing device that integrates with the systems master microprocessor. This advanced system also includes our patented visual inspection system, the VIU
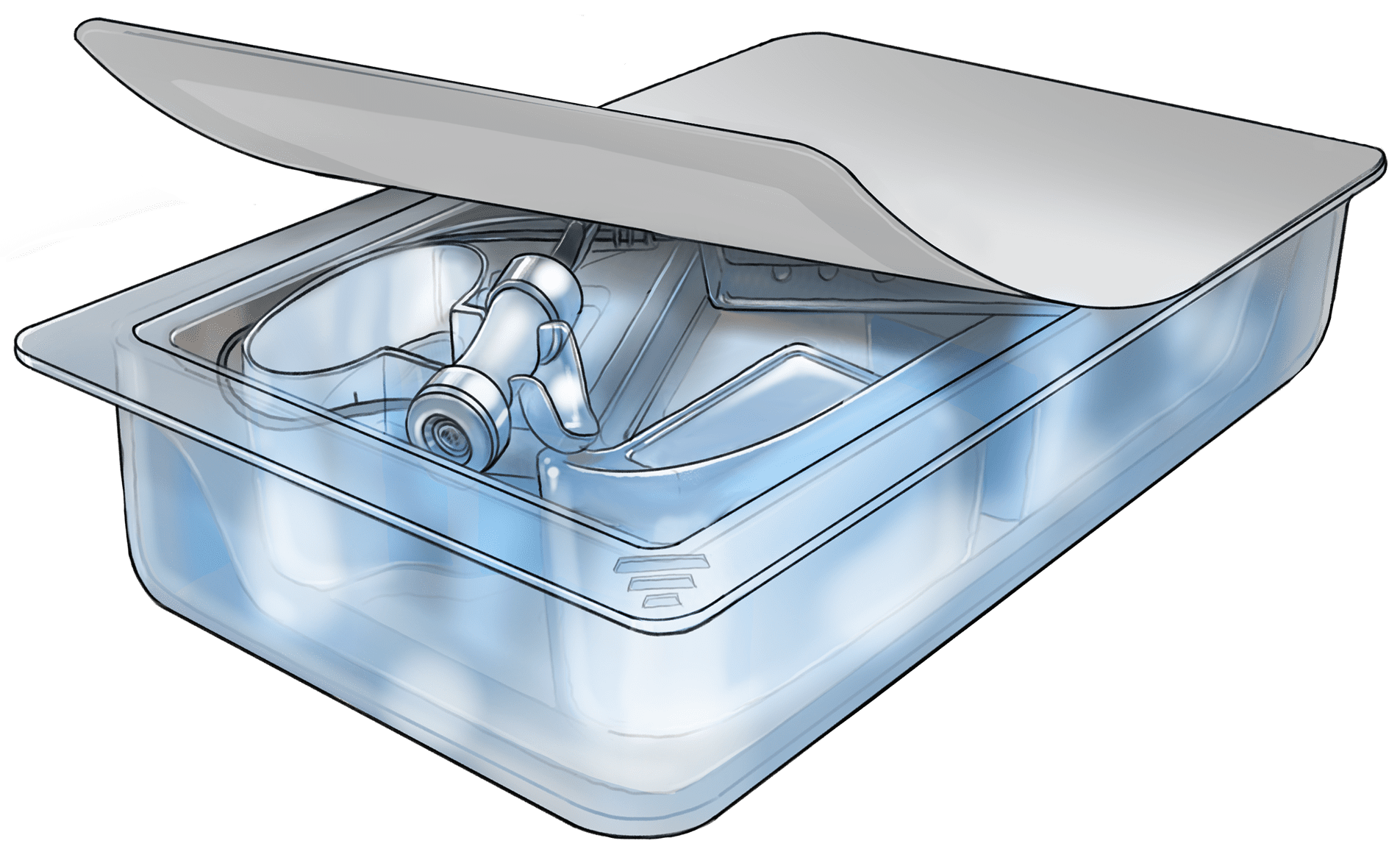
Cross-Contamination solutions
When processing human tissue, cross-contamination is an always present threat. With our patented HTIP system (human tissue isolation pouch) we isolate your packaging process to help thwart cross-contamination between donor events. The HTIP system helps prevent machine contamination and increases medical device packaging machine component life cycles, saving money while managing infection risks to the patient.
Pouch Seal Visual Inspection
The VIU™ can be placed in your cleanroom at the point of packaging to encourage regular visual inspection. Our patented low-angle lighting source casts a light wash over the sealed area of the pouch to better reveal peaks on the sealed surface that could indicate a seal risk. With the provided visual inspection guide operators can compare known issues with the results of their inspection to better isolate the failure type.
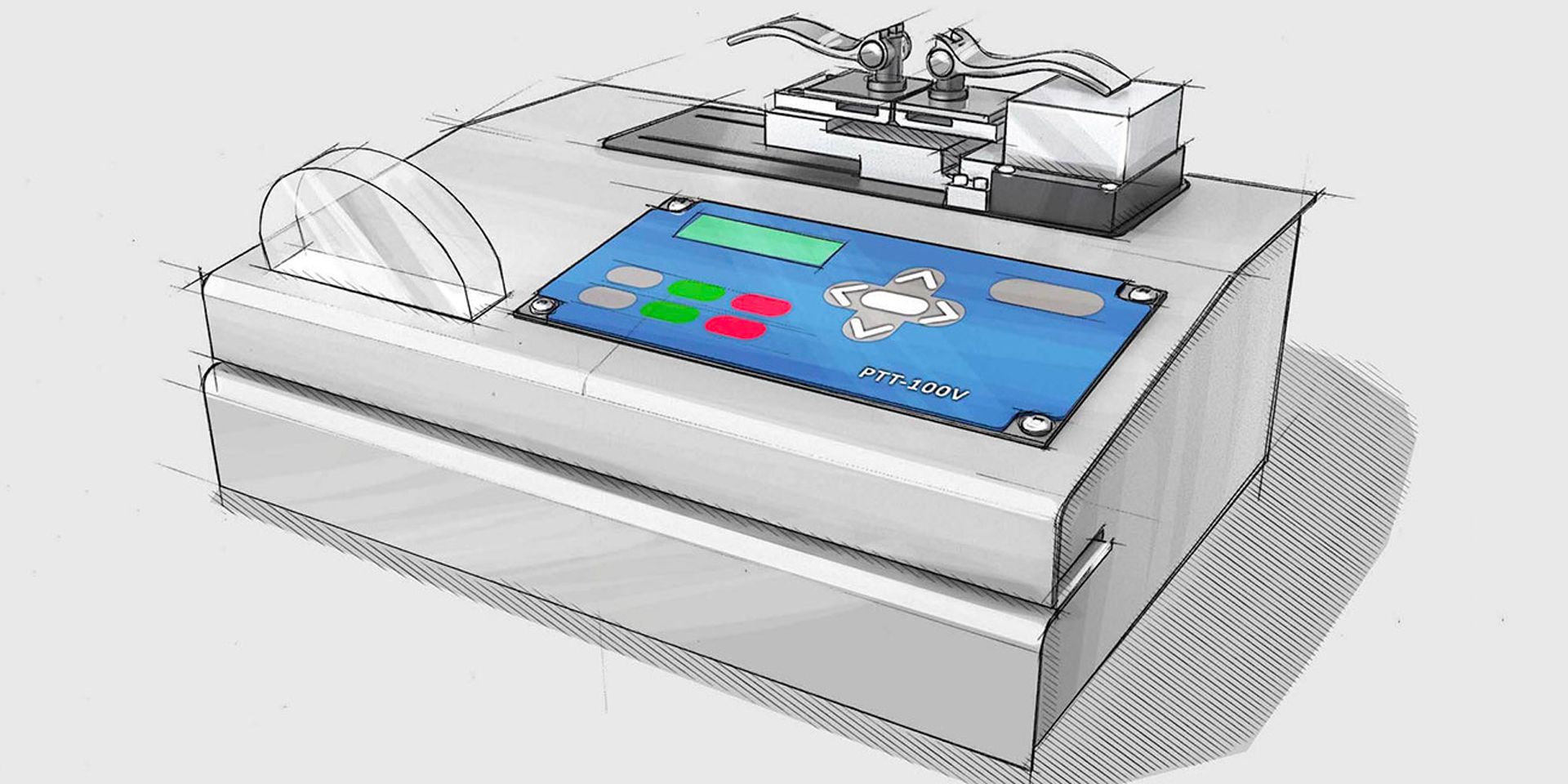
Pouch testing, and inspection
Why risk a potential medical packaging recall? Pouch testing and inspection is the most vital piece of your sterile device packaging plan. With our PTT-100V medical pouch testing and inspection lab, you can thwart compromised pouches from reaching the sterile field. The system features “Quick load” grips for facet loading. Your PTT-100V ships with a full ISO 17025 calibration from our lab. Stay compliant and manage recall risks.