Machinery for your zero defect value…
SterileAware® takes a fresh approach to solving healthcare packaging problems with education, awareness, and innovation.

Machinery for your zero defect value…
A single seal failure on your medical pouch is unacceptable. Choose packaging machinery with a focus on patient safety.

Category-leading medical
device packaging machines…
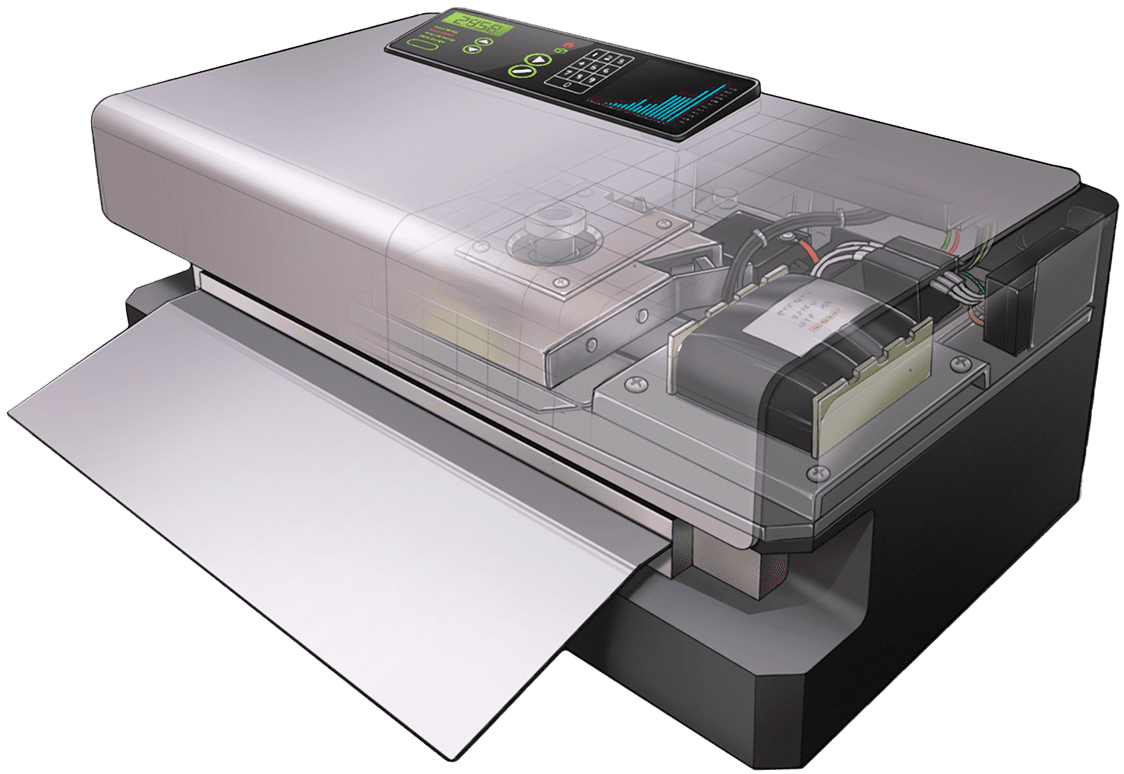
The sterile aware mission to deliver medical devices safe and sterile to the point of care begins with our partnership with our parent company Van der Stähl Scientific. This partnership allows us to bring patented medical device pouch sealing systems to manufacturers so they can be assured that their medical devices will reach the point of care safe and sterile. Packaging systems like our patented MS-451 are changing the way device makers package, watch our video now and learn more.
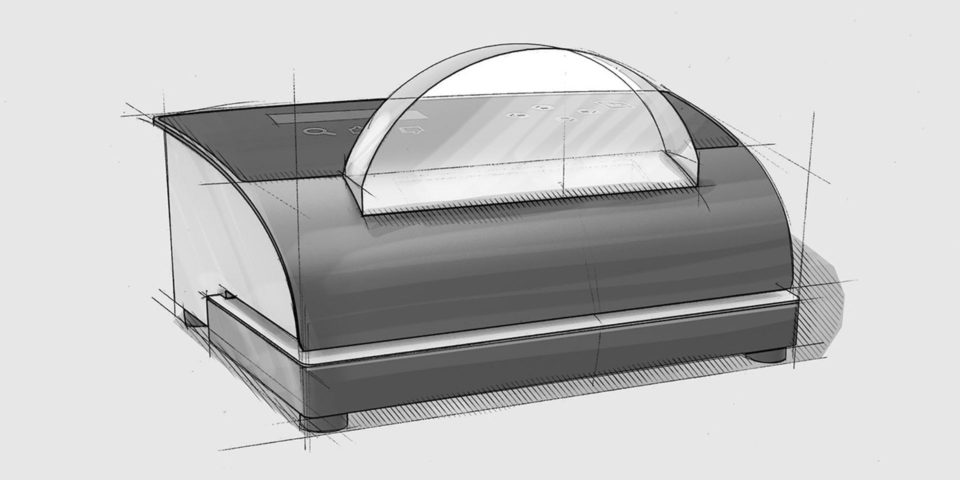
MD-880 medical device pouch sealer
Our rotary sealer allows operators to feed their medical device pouches rapidly through the feed system for an impressive throughput. The MD-880 medical device rotary sealer can also print on the Tyvek® side of your pouch with important information such as expiry dating and lot numbers. We include a calibration from our award-winning ISO 17025 calibration Laboratory as well as a host of packaging validation support.
MS-350 medical device pouch sealer
For 27 years we have provided the industry with the most advanced medical device pouch-sealing machinery. We have sold thousands of MS-350 sealers around the world and our customers continue to buy this workhorse medical pouch sealer for its accuracy and robust build. The sealer comes with calibration from our award-winning ISO 17025 calibration Laboratory as well as a host of packaging validation support.
A complete pouch testing
Laboratory on your benchtop
Experts agree the best way to prevent a medical device packaging recall is with regular pouch strength testing and visual inspection. With our patented PTT-100-V medical pouch testing and inspection system, you can quickly evaluate the Quality of your medical pouch. This intelligent system includes a self-test feature to ensure that the device is in calibration and providing valuable data. Why risk a packaging recall? Test and regularly inspect with the PTT-100-V desktop lab.
Medical device pouch visual inspection
The human eye is an amazing part of our anatomy. With visual inspection, we can discern a host of medical device pouch sealing failures when used in concert with our VIU (visual inspection device). This device allows operators to have a repeatable lighting and magnification source, to better baseline the visual inspection process. With our failure index, operators can associate our images with potential visual seal failures.
Compliant premixed dye solution
ASTM F1929-15 ready premixed medical device pouch test solution for rapid testing. Now medical device packagers can purchase our premixed ink in bulk quantities. For our customers with a more demanding workflow that requires multiple dye penetration testing events throughout the day, we now offer a premixed solution in 8oz, 16oz, as well as a 32oz sizes. The dye solution comes in FDA-compliant Boston bottles.
Safe and sterile device delivery is our promise to the patient…
Sterile packaging support
SterileAware® supports our medical device packaging customers with our Award-winning ISO 17025 accredited laboratories. Empirical pouch testing data is the most valuable component of your packaging validation. Let us help support your DOE through third-party ISO 17015 accredited testing.
ISO 17025 empirical laboratory services
Let us evaluate your medical device packaging by utilizing the ASTM F-88 seal strength test as well as the ASTM F-1929-15 dye penetration test. Our ISO 17025 accredited laboratory provides you easy to interpret high-resolution reporting that provides high value to your audit pathway. Our testing technicians are quality system certified to assure the most accurate testing. Contact our engineering group today and let’s get started.
The value of accredited Calibrations
Most providers of medical device packaging machinery require you to send your machinery to a third-party lab for calibration. We support our medical device manufacturers with two ISO 17025 accredited laboratories that create a seamless connection between the machine and your regulatory needs. The SterileAware® value means purchasing critical packaging machinery from firms that support your regulatory requirement.